
Particles have the same electronic structureġ) Na 0 and Na + 2) Na 0 and K 0 3) Na + and F - 4) Cr 2+ and С r 3+įorms an element with an electronic configuration c the outer electron layerġ) ns 2 np 1 2) ns 2 np 3 3) ns 2 np 4 4) ns 2 np 6Ģ1. The number of valence electrons in manganese isġ9. For a sulfur atom, the number of electrons at the external energy level and the charge of the nucleus are equal, respectivelyġ) 4 and + 16 2) 6 and + 32 3) 6 and + 16 4) 4 and + 32ġ8. Element that matches higher oxide composition R 2 O 7 has an external level electronic configuration:įorms a chemical element, in the atom of which the filling of energy levels with electrons corresponds a number of numbers:ġ) the number of valence electrons in atoms increasesĢ) the number of electronic layers in atoms decreasesģ) the number of protons in the nuclei of atoms decreasesġ6.Electronic configuration 1 s 2 2 s 2 2р 6 3.s 2 Зр 6 3 d 1 has an ionġ7. A metal atom, the higher oxide of which is Me 2 O 3, has an electronic formula for the external energy levelġ1. The same electronic configuration of the external level has Ca 2+ andġ0. Electronic configuration Is 2 2 s 2 2 p 6 3 s 2 3 p 6 corresponds to ionĩ. Element with electronic configuration of external level.
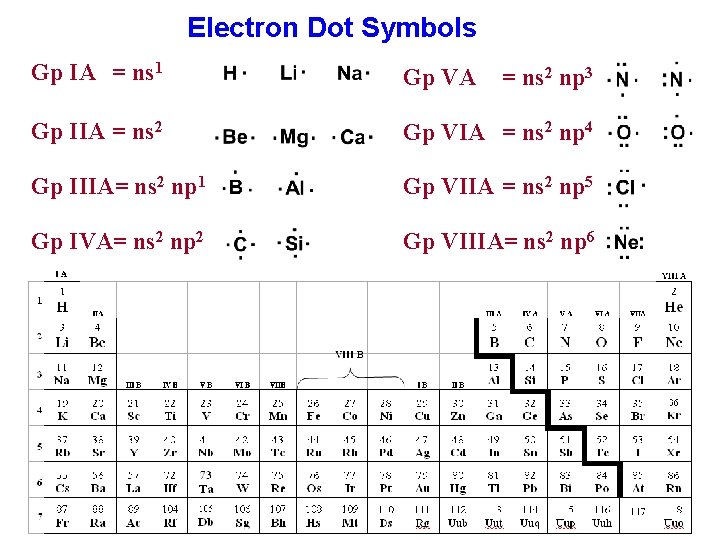
In the ground state, three unpaired electrons have an atomĦ. The number of electrons in the iron ion Fe 2+ isĥ. The two-electron outer shell has an ionģ. The eight-electron outer shell has an ionĢ. Metals are found on the left of the line, whereas non-metals are those on the right. The crisscross line which is present in the P-Block isolates metals from non-metals.

The P-Block comprises metals, non-metals, and metalloids. For all these reasons, it is in the P-Block.
NS2 NP3 FULL
It is much less reactive like other inert gases, and its chemical properties are more alike to the P-Block noble gases in group 18 because of a full shell. In spite of having the configuration of S-Block elements, Helium is closely related to the noble gases. What is the easiest way to memorize the periodic table? How do you remember the P-Block elements?įor Group 13 -‘Brother Andrew got intelligent thought’ stands for Boron, Aluminium, Gallium, Indium, and Thallium, respectively.įor Group 14 – ‘Can Son go to left’ stands for Carbon, Silicon, Germanium, Tin, and Lead, respectively.įor Group 15- ‘Nobody passed away answer back’ which stands for Nitrogen, Phosphorous, Arsenic, Antimony, and Bismuth.įor Group 16 – ‘Oh somebody should tell possible’ stands for Oxygen, Sulphur, Selenium, Tellurium, and Polonium.įor group 17 – ‘Fish can’t breathe in air’ stands for Fluorine, Chlorine, Bromine, Iodine and Astatine.įor Group 18 – ‘He never arrived kid xeroxed randomly’ which stands for Helium, Neon, Argon, Krypton, Xenon and Radon.ģ. Here, the valence electrons are in the p orbital.Ģ. The P-Block is located on the right side of the periodic table and comprises elements from 13 to 18.
NS2 NP3 FREE
These are found in both free and combined states in nature. It consists of the elements oxygen, selenium, sulfur, tellurium, and polonium. The Oxygen Family is also called the chalcogens, consisting of the elements in Group 16. Generally, family elements are stable and tend to be somewhat unreactive.

Atoms in this group have four valence electrons. The Carbon Family is group 14 of the periodic table consisting of five elements: carbon, silicon, germanium, tin, and lead. They have three electrons in their outermost shell with a full s orbital and one electron in the p orbital and the valence electron configuration is ns2np1. The Boron Family contains elements in group 13 of the periodic table, including the semi-metal boron and the metals aluminum, gallium, indium, and thallium. All elements in the P-Block are silvery solids except element boron, which is brown solid.But as we move down in a group, the chemical reactivity of elements declines. The chemical reactivity of elements in the P-Block increases as we move to the right in a period.The P-Block elements generally have a positive anode potential, and part of it decreases down the groups.The atomic radius increases highly from Boron to Aluminum. The ionic radius increases down the group as one additional shell gets added to the element with an increase in the atomic number.The oxidation states increase as we move from left to right in the periodic table.The atomic density of elements in P-Block increases down the group due to an increase in the atom’s size down the group.The metallic nature tends to increase moving down every group while it declines as we go from left to right.


 0 kommentar(er)
0 kommentar(er)
